|
2.
The synthesis process to obtained one-dimension (1D) BaTiO3
nanostructures it is optimized; Structural and microstructural
characterization mainly by electron microscopy techniques; Synthesis
parameters studied: reaction temperature and mineralizer concentration
|
|
|
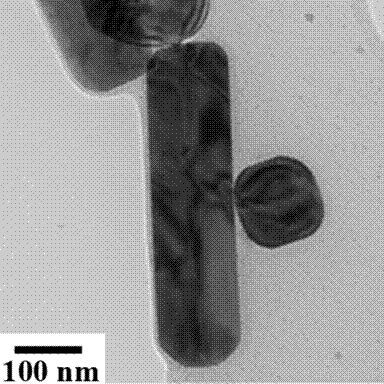
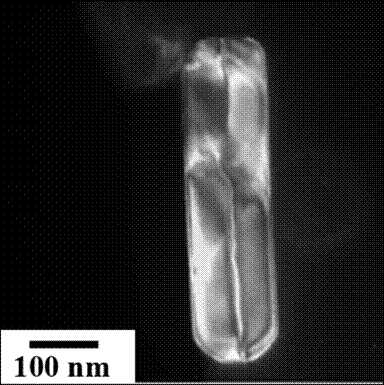
Bright field (left) and dark field
(right) TEM images of BaTiO3 1D nanorods obtained at 200 °C and 10 M mineralizer
concentration; In the dark field image the ferroelectric domains along
the rod axis are illustrated.
|
.: 2013 Scientific Report
1. The synthesis process to obtained
BaTiO3 nanostructures it is optimized; Structural and
microstructural characterization mainly by electron microscopy
techniques; Synthesis parameters studied: barium and titanium precursors
|

|

|
SEM
images of the BaTiO3 hydrothermal powders showing the formation
of hollow particles when using titanium based nanotubes as titanium
precursors and barium chloride as barium source. The relations between
processing, structure and morphology development of the BaTiO3
nanostructures has been established; The growth mechanism of the hollow
BaTiO3 particles was attributed to the Kirkendall effect (http://dx.doi.org/10.1002/ejic.201402497).
|
2.
Synthesis of monophase BiFeO3 powders by hydrothermal method
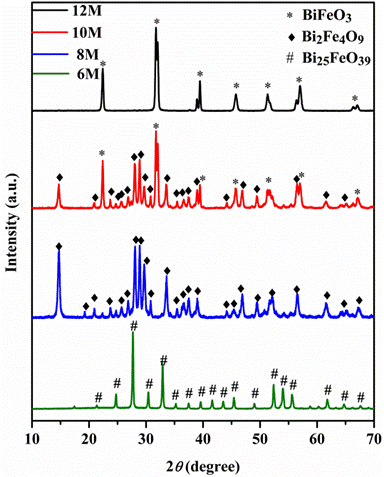
XRD patterns of the hydrothermal powders showing that perovskite
phase BiFeO3 is obtained for 12 M mineralizer
concentration
3.
Electrical and thermodynamic characterization of the obtained
nanostructures
|
|
|
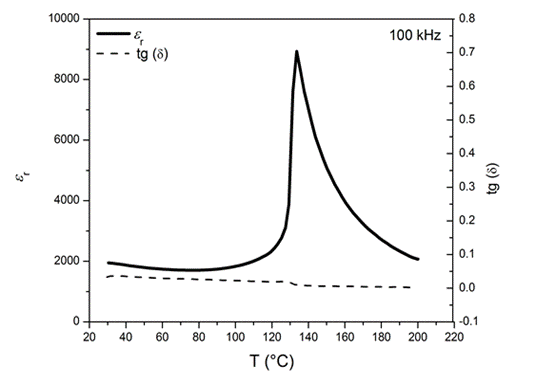
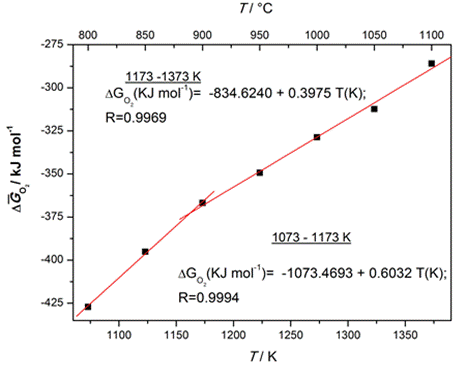
(Left side) Electric
properties: Dielectric constant and dielectric
losses as a function of temperature for the BaTiO3 ceramics
obtained from the hydrothermal powders; (Right side) Thermodynamic
properties: The relative partial molar free energies of the oxygen
dissolution in the perovskite BaTiO3 phase.
|
|