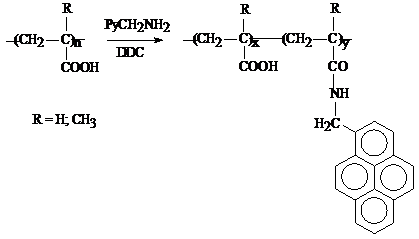
The hydrophobically (fluorescently and with an alkyl-chain label) modified polymers were synthesized following recipes published elsewhere [D.F. Anghel, V. Alderson, F.M. Winnik, M. Misuzaki, Y. Morishima, Fluorescent dyes as model ‘hydrophobic modifiers’ of polyelectrolytes: a study of poly(acrylic acid)s labelled with pyrenyl and naphtyl groups, Polymer, 39 (1998) 3035-3044; B. Magny, Polyelectrolytes Associatifs: Methodes de Synthese, Comportement en Milieu Dilue et Semi-Dilue, These de Doctorat, 1992.]. The chemical reactions involved were:
1) for pyrene and naphthalene labeled polymers:
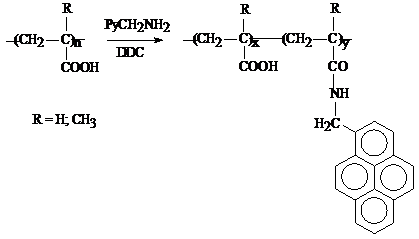
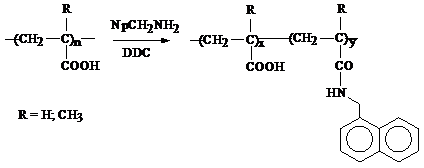
2) for hydrophobically modified polymers with different alkyl chain lengths:
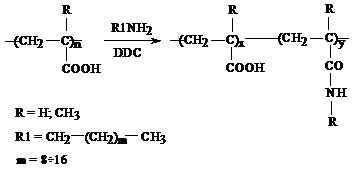
The characterization of the labeled polymers was done by different methods. FT-IR spectroscopy evidenced the chemical modification of polyacrylic acid (PAA). The bands at 1624 cm-1 and 1551 cm-1 (shoulders of the carbonyl band from PAA) correspond to amide I and amide II vibrations, proving that the polymer analogous reaction occurred successfully. These characteristic vibrations can be observed both in the case of fluorescently and hydrophobically modified PAA (PAA-Py and PAA-C12, respectively - see Figure 2012-1).
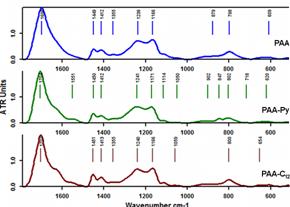
Figure 2012-1. FT-IR spectra of PAA, PAA-Py and PAA-C12.
The content of pyrene grafted on polymeric acids was determined by UV-Vis and 1H-NMR. 1H-NMR spectroscopy offers quantitative information regarding the modification degree of PAA. In the case of PAA-Py sample, we determined the modification degree based on the integral corresponding to pyrene aromatic protons (8-8.5 ppm) and the integral corresponding to PAA backbone protons (1-2.4 ppm). To calculate the modification degree for PAA-C12 we used the signal corresponding to CH3 (0.86 ppm) protons from the alkyl chain of the amide, the signal between 1-2.4 ppm corresponding to PAA backbone protons, and the methylene protons (d protons see Figure 2012-2) from the dodecyl chain.
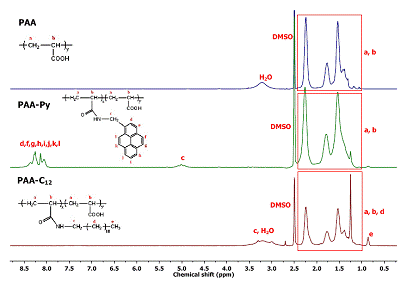
Figure 2012-2. The 1H-NMR spectra of PAA-Py and PAA-C12 and, for comparison, that of the parent PAA.
The UV-Vis and 1H-NMR revealed that the modification degree was of 3 mol % for both PAA-Py and PAA-C12.
The GPC analysis proved that the synthesized polymers did not contain small molecular impurities which absorb in UV. Also, the method revealed that the chemical modification did not alter the molecular weight of PAA precursor.
The study of photophysical properties of the fluorescently labeled polymers gives information about the phenomena occuring at molecular level in self-assembled polymer systems. The pyrene labeled PAAs are pH or other external stimuli responsive polymers. The intensity ratio of the first to the third vibronic peaks (I1/I3) of pyrene label offers information on the polarity of its microenvironment. Besides, the excimer to the monomer intensity ratio (IE/IM) gives information on the random coil. The steady-state fluorescence measurements were done at different pH, on fluorescently labeled polymers with various molecular weights. The results revealed that at low pH the label sensed a more nonpolar microenvironment than at high pH. The reason is that, at low pH, the polymer coil is shrinked, and the fluorophore locates inside. The IE/IM ratio decreased with increasing pH. This is due to the increasing of the electrostatic repulsions between the carboxilic groups which leads to polymer unwinding.
Additional information was obtained by dynamic fluorescence measurements. The decay profiles of PAA-Py was measured at the monomer (lex = 374 nm) and excimer (lex = 480 nm) emissions. The decays were multi-exponentials (see Figure 2012-3) indicating the existence of more emissive species. Also, it was observed that, as pH raised, the fluorescence lifetimes decreased, proving that the polymer uncoils at alkaline pHs.
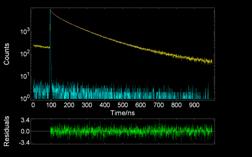 |
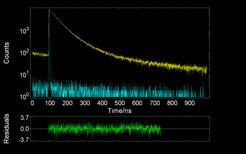 |
Figure 2012-1. FT-IR spectra of PAA, PAA-Py and PAA-C12
Grafted polymers respond to external stimuli (pH, surfactants, etc.) and gives information on the conformation of the statistical coil and on the environment in which they are solubilized. A method largely used to investigate the phenomena happening at nanometric level between two fluorophores is the non-radiative energy transfer (NRET). NRET occurs when a donor (D) in its excited state transfers energy to a ground state acceptor (A), the two florophores being in close proximity. For characterization the micellar interface we applied NRET, using naphthalene (Np) probe confined in surfactant micelles as donor and grafted pyrene (Py) on polyacrylic acid (PAA-Py) dissolved in water as acceptor. We determined the NRET efficiency between naphthalene probe and PAA-Py in micellar solutions at variuos pH values. The calculated Förster radius and the donor-acceptor distance were within the Förster theory. Supplementary data were obtained by UV-Vis spectroscopy, surface tension and dynamic light scattering (DLS) measurements. Micelles were prepared with anionic [sodium dodecyl sulphate SDS and bis-(ethylhehyl) sodium sulfosuccinate (AOT)] and nonionic [decaethylene glycol monotetradecyl ether (C14EO10)] surfactants. NRET experiments were conducted at pH values between 2 ÷ 11. The results are presented in Figure 2013-1. In these systems, the pyrene emission appears beyond 360 nm as a consequence of non-radiative energy transfer from naphthalene excited at 290 nm and is strongly dependent on pH.
A qualitative measure of energy transfer for studied systems is the IPy/INp ratio. A high value of it reflects high efficiency of non-radiative energy transfer and reverse. For anionic SDS and AOT surfactants, IPy/INp decreases with pH (Figure 2013-2). Increasing the pH causes the transformation of carboxyl groups into carboxylate groups, and PAA acquires more negative charges and adopts a more open conformation. This conformation has many negative charges that repel anionic micelles and NRET decreases. For the C14EO10 – PAA-Py system, it was initially observed a gradual decrease in IPy/INp values, until pH = 4.24 when a minimum is reached, then IPy/INp values increased and has a plateau above pH 7. The increase of NRET can be explained taking into consideration the non-cooperative and cooperative binding of the surfactant molecules onto the polymer. They produce polymer uncoiling, and drives out the pyrene labels in water. If Py encounters a surfactant micelle containing excited Np, the IPy/INp will increase. The number of Py labels available for energy transfer becomes constant and explains the plateau above pH 7.
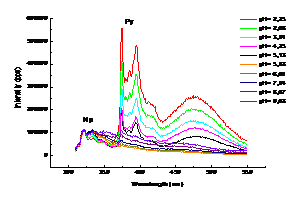
Figure 2013-1. Non-radiative energy transfer between Np and PAA-Py in micellar AOT–PAA-Py systems at different pH values.
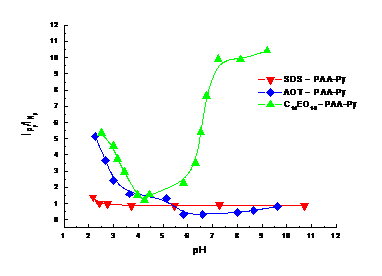
Figure 2013-2. Effect of pH on IPy/INp ratio for surfactant- PAA-Py systems. λex= 290 nm.
The average distance between donor and acceptor (calculated by Förster theory), r, is within the 25.0 – 32.9 Å range and obeys the relation 0.5R0 < r < 2R0, where R0 is the critical Förster distance at which 50% of the excitation energy is transferred to the acceptor. For the anionic surfactants r varies in the order: AOT > SDS. The largest distance for AOT may be due to the bulky hydrophobic group that hinders the polymer interaction with the micelle of this surfactant. In the system with C14EO10, the smallest value of r was found at pH = 7.22. At this pH, the PAA coil unfolds and exposes the hydrophobic pyrene labels to water, allowing them to interact with the surfactant micelles containing the solubilized naphthalene.
The efficiency of non-radiative energy transfer, E, was calculated by measurements of donor intensity at 319 nm, in the absence and presence of acceptor. In all studied micellar systems, the spectral overlap integral has the same order of magnitude. For the system with anionic surfactants, the energy transfer efficiency is higher for SDS than for AOT. Transfer efficiency is lower when using anionic surfactants than the nonionic C14EO10. Moreover, the transfer efficiency in nonionic surfactant system depends on the pH and increases when pH changes from acid to neutral. The values of the donor quantum yield (ΦD) in the studied systems are similar. The highest ΦD is obtained in AOT micelles, and it is in conformity with the branched structure of this surfactant that entails a more loosened packing of the hydrophobic tails into its micelles than in those of SDS that have a linear alkyl chain. This allows to the solubilized Np to come in the vicinity of the Py label at the micelle surface and to interact to each other.
Polymers nanofilms are widely used for the modification and functionalization of the surfaces. The polyelectrolyte multilayer depositions were accomplished by layer-by-layer (LbL) technique on slides. This technique is a versatile method to obtain stratified ultrathin films and has several advantages like easiness of fabrication and the availability of various polyelectrolytes.
The LbL deposition is a thin film fabrication technique. The films are formed by depositing alternating layers of oppositely charged materials with wash steps in-between. In our case, the film is deposited on quartz surface by dipping succesively the slide into beakers containing polycation and containing polyanion solutions (Figure 2014-1).
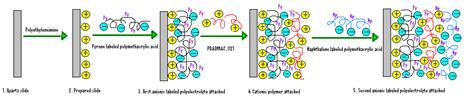
Figure 2014-1. Layer-by-layer technique.
As anionic polyelectrolytes we used polymethacrylic and polyacrylic acid grafted with pyrene and naphthalene (PMA-Py, PMA-Np, PAA-Py, PAA-Np). The cationic polyelectrolyte was polydialyldimethylammonium chloride (PDADMAC). For the intermediate layers that do not contain fluorophores we used sodium salt of polymethacrylic and polyacrylic acid. The obtained multilayers were characterized by absorption and fluorescence spectroscopy. Complementary data on the behaviour of grafted polyelectrolytes in solution were obtained by dynamic light scattering (DLS). Hydrodynamic diameters of the fluorescently grafted polyelectrolytes decrease in the presence of salt, which leads to thicker films.
The obtained NRET data on our films reveal that the non-radiative energy transfer occurs both in the films where donor (naphthalene graft) and acceptor (pyrene graft) layers are deposited successively, and also in the films where the donor layer is separated from the acceptor by many photophysical inactive bilayers.
This study continues the previous step regarding the obtaining of polyelectrolyte multilayers by layer-by-layer (LbL) technique. Our aim for the present stage is to obtain hydrophobic films with the aid of hydrophobically modified polymers, with and without cationic surfactants of variable alkyl chains (decyl-, dodecyl-, tetradecyl- and octadecyltrimethyl ammonium bromide, C10-, C12-, C14-, C18TAB). Poly(acrylic acid)s (PAA) grafted with decylamine (PAC10Na), and respectively with octadecylamine (PAC18Na) were synthesized, purified and characterized. Films from aqueous solutions of the above systems were deposited on glass slides by LbL procedure and were characterized by contact angle measurements and microscopy (AFM, etc). To allow the successive deposition, the cationic polymer [poly(diallyldimethylammonium chloride), PDADMAC] was intermediary introduced.
One observed that the contact angle increases with the number of bilayers and with surfactant hydrophobicity (see Figure below). The films containing PAC10Na-C18TAB are more hydrophobic than those with PAC10Na-C10TAB.
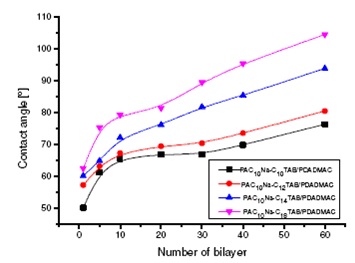
Figure 2015-1. The contact angle variation with the number of bilayers
for the PAC10-CxTAB/PDADMAC films.
AFM results show non-uniform structures that confer an improved hydrophobicity to the surfaces, confirmed also by the contact angle data.
The results evidence the effect of the hydrophobic character of the modified polymers and surfactants upon the wettability and roughness of the obtained films.